How Many Valence Electrons Are in the Neon Family
Jun 25 2017 The members of the boron family have 3 valence electrons. We know the electron configuration of the Neon atom is 1s 2 2s 2 2p 6 and valence electrons are those electrons found in the outer shell of an atom.
How Many Valence Electrons Are In Fluorine Quora
Which group has all 7 valence electrons.
. These elements already have a full outer energy level so they are very stable. He its valency will be 3. Therefore the valence electrons of neonNe are eight.
Since neon is a noble gas it has 8 valence electrons. Four other pairs of elements in the same chemical family are listed below. Group 13 has 3.
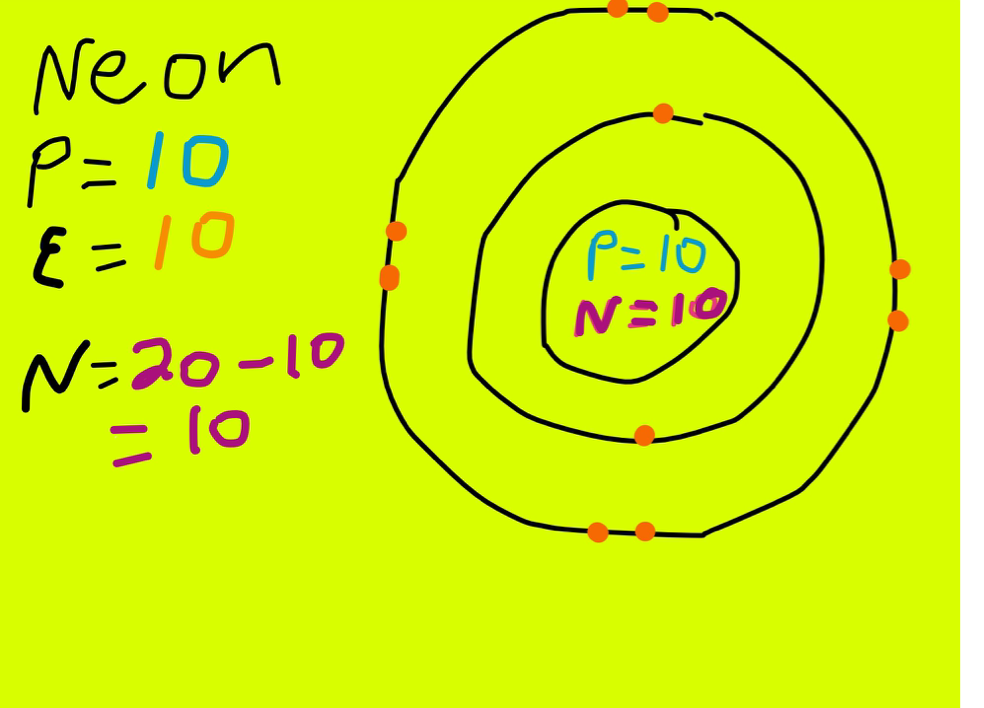
How many valence electrons does the noble family have. Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. And thus the neutral atom has 7 valence electrons.
The total number of electrons in a valence shell is called a valence electron. There are a few with 11 neutrons and about 9 with 12. Neon-20 is composed of.
There are 8 electrons in the valence shell of neon The. Thus neon has eight valence electrons. The Noble Gases have electronegatives.
Neon has 10 electrons 2 in the first shell and 8 in the second shell so eight valence electrons. The electron configuration of argon shows that the last shell of argon has eight 3s 2 3p 6 electrons. Any element in the halogen group will have seven valence electrons.
Group 1 has one valence electron. Eight valence electrons Noble gases are the least reactive of all elements. These elements include fluorine chlorine bromine iodine and astatine.
90 of neon atoms contain 10 neutrons. Therefore the valence electrons of argon Ar are eight. What is the valence shell of neon letter.
The group number of the boron family indicates the number of valence electrons. Valence Electrons and Electricity 1. Elements in other groups have partially-filled valence shells and gain or lose electrons to achieve a stable electron configuration.
How many valence electrons would you find for elements in each family. Of electronsshell36krypton2 8 18 854xenon2 8 18 18 886radon2 8 18 32 18 8. The total number of electrons present in the valence shell of an atom is called valence electrons and there is only one electron present in the valence shell of neon 2s²2p⁶.
How do you think the number of valence electrons relates to an. How do you find the valence electrons of F. The Valence electron level is full or the Noble Gas family.
Group 15 has 5. Because neon has all 8 electrons in the outermost shell which is balance So neon element doesnt need to share or take any electron from any other one so its valency is zero. The last shell after the electron configuration is called the valence shell.
Group 2 has 2. Valence Electrons for Argon Ar. This electron configuration of Neon shows that the outer shell of Neon has 8 electrons 2s22p6 hence the number of valence electrons in the Neon atom is 8.
Each shell has a certain amount of subshells s p d etc. Group 14 has 4. The shells of an atom can only hold so many electrons.
23092021 Neons outermost shell has 8 electrons. There are 10 protons and 10 electrons in non-ionized neon. Neon along with helium argon krypton and xenon make up the group known as noble gases.
So that the valency of boron B is 3. How many neutrons are there in a neon atom. Neon has three stable isotopes.
The valence electrons in the members of the boron family are in the highest energy s and p orbitals in particular s2p1. True there are 8 electrons in the valence level making it full. Neon is the second lightest inert gas.
Valence electrons in Nitrogen N 5. Helium Neon Argon Krypton Xenon and Radon. Elements in other groups vary in their reactivity but are generally less reactive than elements in groups 1 2 16 or 17.
Neon therefore has 8 valence electrons. This closed shell configuration makes neon supremely difficult to oxidize and difficult to reduce. Neon Z10 has eight valence electrons.
Valency of Neon Ne. Boron has both 3 -3 valency states. How many valence electrons are in each group of the main group.
Upon reduction the fluorine atom forms fluoride which has 8 valence electrons and is isoelectronic with a Noble Gas which one. 20Ne 9048 21Ne 027 and 22Ne 925. The group 18 elements helium neon argon krypton xenon and radon noble-gas configuration.
Thats because they have eight valence electrons. Which family has a full outer energy level. And 10 electrons.
How many valence electrons does this element have. How many orbitals are in neon. The group number is 13IIIA.
Noble gases have eight electrons in their outermost shell except in the case of helium which has twoElectron configurationZElementNo. Those with extra neutrons are called isotopes the nuclei having 10 protons but a few with a different number of neutrons. The electron configuration of neon shows that the last shell of neon has eight electrons2s 2 2p 6.
If it loses three electrons to reach a stable state ie. The last shell after the electron configuration is called the valence shell. A full valence shell is the most stable electron configuration.
The total number of electrons in a valence shell is called a valence electron. How many valance electrons are found in an atom what elements Neon. An outer main energy level fully occupied in most cases by eight electrons.
Eight electrons Neon like all noble gases has a full valence shell. Does neon have a full valence shell. Group 18 elements helium neon and argon are shown have a full outer or valence shell.
Of course the elemental form is bimolecular. Valence electrons in Oxygen O 6. Elements like boron can reach the stable state nearest inert gas configuration by losing 3 outermost electrons or by getting 5 electrons.
Atoms of group 18 elements have eight valence electrons or two in the case of helium. For example the atomic number of Ne neon is 10 and contains 5 orbitals 1s 2s 2p x 2p y and 2p zIn each full orbital there are 2 electrons giving a total of 10 to balance the positive charge provided by the 10 protons in the nucleus.
Which Element In The Periodic Table Has 5 Orbital Shells And 5 Valence Electrons Quora
How Many Valence Electrons Does Cl Have Quora

How Many Valence Electrons Does Neon Ne Have

Neon Orbital Diagram Electron Configuration And Valence Electrons
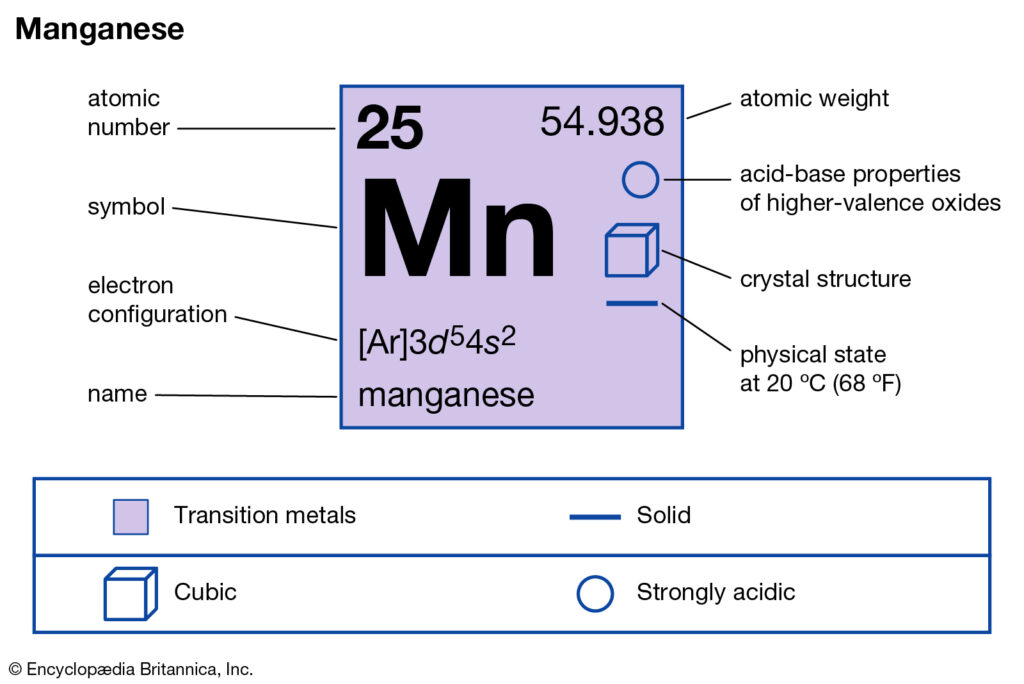
Manganese Valence Electrons Manganese Valency Mn Dot Diagram
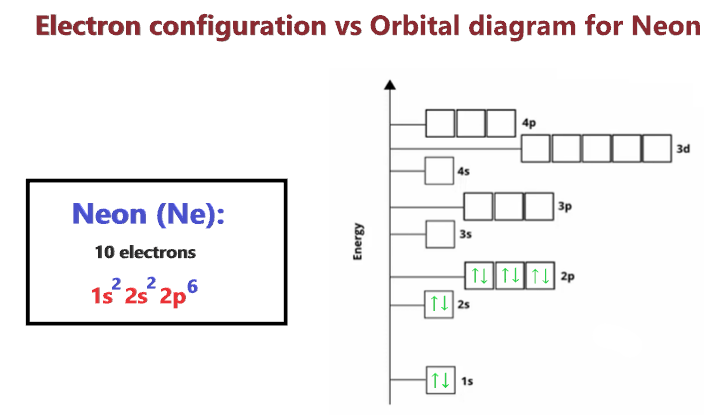
Neon Orbital Diagram Electron Configuration And Valence Electrons

Neon Family Valence Electrons Octalcomics

Element Families On The Periodic Table
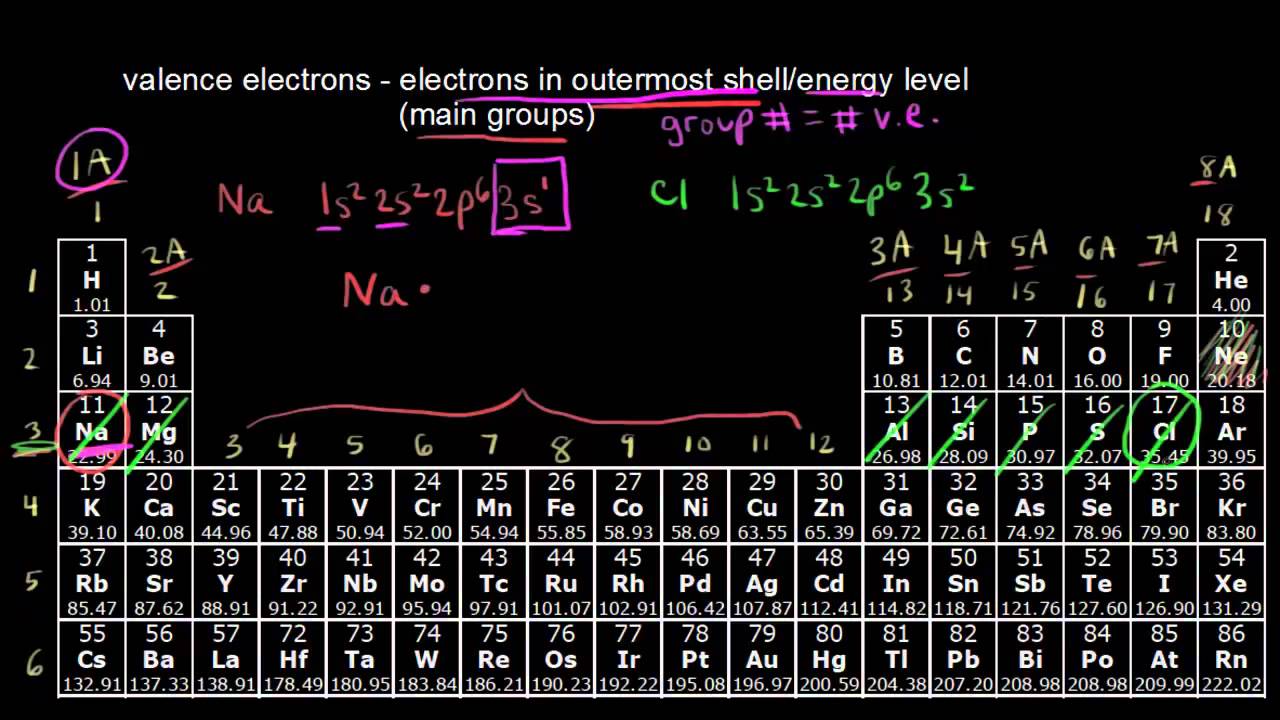
Counting Valence Electrons For Main Group Elements Video Khan Academy

How Many Valence Electrons Does Neon Ne Have

Neon Orbital Diagram Electron Configuration And Valence Electrons

The Noble Gases Group 18 Introduction To Chemistry

Neon Orbital Diagram Electron Configuration And Valence Electrons

How Many Valence Electrons Does Neon Ne Have

Chemistry291 Hand Note 5 Steps How Many Valence Electrons Does Sodium Electrons Electron Configuration Chemical Equation

Valence Electrons 1 7 How Do Electrons Determine Chemical Behavior Ppt Download

Neon Project Screen 7 On Flowvella Presentation Software For Mac Ipad And Iphone

Comments
Post a Comment